Advanced brain tumor evaluation tools - at your fingertips
The tumor evaluation package includes everything you need to assess and diagnose brain tumors. Our most powerful image analysis platform yet, combined with tools and algorithms, makes for a truly seamless, efficient and accurate image analysis workflow.
Perfusion: DSC and DCE
We have included both DSC and DCE perfusion capabilities in this package. The DSC analysis can be set up to be completely automatic to rapidly generate maps of CBV and CBF and our patented automatic normalization algorithm provides the ability to perform an AIF-free approach when estimating these parameters.
The DCE processing features generation of Ktrans, ve, and vp using the Tofts and the Extended Tofts models and provides quantitative measurements of tumor vascularity.
Diffusion
In addition to diffusion tensor imaging and spherical deconvolution, we can analyze DWI to generate ADC maps.
Automation and standardization
In nordicMEDiVA, you can define the analysis pipelines and settings organization- wide, making the image analysis pipelines standardized across the organization. You can define multiple pipelines depending on the use case, and even limit who has access to add and modify analysis pipelines.
We follow the recommendations set out by the Quantitative Imaging Biomarker Alliance for DSC and DCE imaging.
-

ROI Analysis
A short description with details about the header.
-
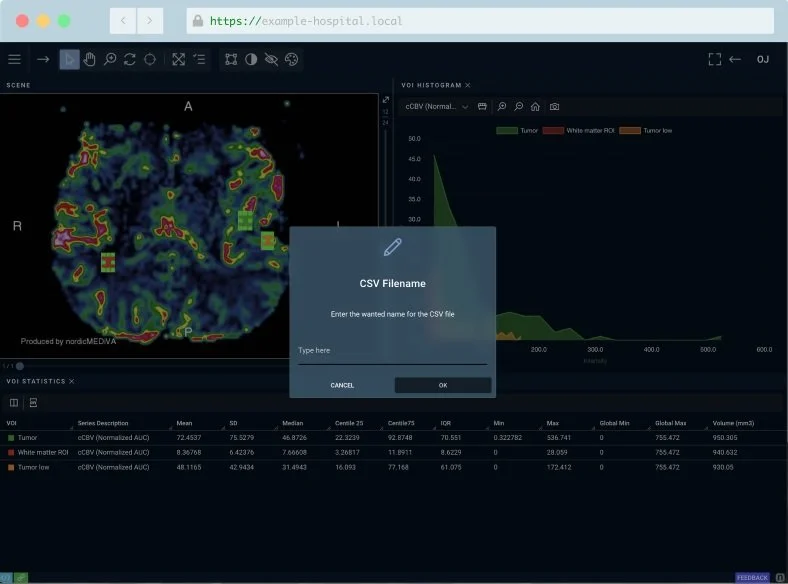
Export ROI Measurements to Excel or CSV
A short description with details about the header.
-

In-app spreadsheet
A short description with details about the header.
More features
-
DSC: CBV, CBF, TTP
DCE: Ktrans, ve, vp
Diffusion: ADC
-
We have included state-of-the-art diffusion processing, as well as well-proven algorithms for DSC and DCE processing.
-
The advanced motion correction algorithm in nordicMEDiVA can correct for motion up to 30 degrees and has been tested on perfusion data. Motion curves are provided as output for quality control.
-
An alternative approach to using AIF deconvolution for perfusion processing is to normalize the perfusion maps to normal-appearing white and grey matter. This eliminates the variability in the choice of AIF and may yield more reproducible results.
This option has been included as standard in the nordicMEDiVA Tumor Evaluation package.
-
Select either Tofts or Extended Tofts model for DCE processing.
-
This feature is not implemented and may never become available for sale.
Load multiple time points from the same patient to get a full treatment history for a complete assessment of the treatment response. By adding automatic tumor evaluation, this analysis can be done effectively and accurately.
Scientific references
Below you will find a list of scientific references, papers, and guidelines we have used in the development of the nordicMEDiVA Tumor Evaluation package.
DSC
-
Abstract
Dynamic susceptibility contrast (DSC)-based perfusion analysis from MR images has become an established method for analysis of cerebral blood volume (CBV) in glioma patients. To date, little emphasis has, however, been placed on quantitative perfusion analysis of these patients, mainly due to the associated increased technical complexity and lack of sufficient stability in a clinical setting. The aim of our study was to develop a fully automated analysis framework for quantitative DSC-based perfusion analysis. The method presented here generates quantitative hemodynamic maps without user interaction, combined with automatic segmentation of normal-appearing cerebral tissue. Validation of 101 patients with confirmed glioma after surgery gave mean values for CBF, CBV, and MTT, extracted automatically from normal-appearing whole-brain white and gray matter, in good agreement with literature values. The measured age-and gender-related variations in the same parameters were also in agreement with those in the literature. Several established analysis methods were compared and the resulting perfusion metrics depended significantly on method and parameter choice. In conclusion, we present an accurate, fast, and automatic quantitative perfusion analysis method where all analysis steps are based on raw DSC data only.
-
Abstract
The presence of macroscopic vessels within the tumor region is a potential confounding factor in MR-based dynamic susceptibility contrast (DSC)-enhanced glioma grading. In order to distinguish between such vessels and the elevated cerebral blood volume (CBV) of brain tumors, we propose a vessel segmentation technique based on clustering of multiple parameters derived from the dynamic contrast-enhanced first-pass curve. A total of 77 adult patients with histologically-confirmed gliomas were imaged at 1.5T and glioma regions-of-interest (ROIs) were derived from the conventional MR images by a neuroradiologist. The diagnostic accuracy of applying vessel exclusion by segmentation of glioma ROIs with vessels included was assessed using a histogram analysis method and compared to glioma ROIs with vessels included. For all measures of diagnostic efficacy investigated, the highest values were observed when the glioma diagnosis was based on vessel segmentation in combination with an initial mean transit time (MTT) mask. Our results suggest that vessel segmentation based on DSC parameters may improve the diagnostic efficacy of glioma grading. The proposed vessel segmentation is attractive because it provides a mask that covers all pixels affected by the intravascular susceptibility effect.
-
Abstract
This QIBA Profile, Dynamic-Susceptibility-Contrast Magnetic Resonance Imaging (DSC-MRI), addresses the measurement of an imaging biomarker for relative Cerebral Blood Volume (rCBV) for the evaluation of brain tumor progression or response to therapy. We note here, that this profile does not claim to be measuring quantitative rCBV due to lack of existing supporting literature; it does provide claims for a biomarker that is proportional to rCBV, which is the tissue normalized first-pass area under the contrast-agent concentration curve (AUC-TN). The AUC-TN therefore has merit as a potential biomarker for diseases or treatments that impact rCBV. This profile places requirements on Sites, Acquisition Devices, Contrast Injectors, Contrast Media, Radiologists, Physicists, Technologists, Reconstruction Software, Image Analysis Tools and Image Analysts involved in Site Conformance, Staff Qualification, Product Validation, Pre-delivery, Periodic QA, Protocol Design, Subject Handling, Image Data Acquisition, Image Data Reconstruction, Image QA, Image Distribution, Image Analysis and Image Interpretation.
https://qibawiki.rsna.org/images/d/d4/QIBA_DSC-MRI_Stage2-Consensus_Profile.pdf
DCE
-
Abstract
Rapid T1-weighted 3D spoiled gradient-echo (GRE) data sets were acquired in the abdomen of 23 cancer patients during a total of 113 separate visits to allow dynamic contrast-enhanced MRI (DCE-MRI) analysis of tumor microvasculature. The arterial input function (AIF) was measured in each patient at each visit using an automated AIF extraction method following a standardized bolus administration of gadodiamide. The AIFs for each patient were combined to obtain a mean AIF that is representative for any individual. The functional form of this general AIF may be useful for studies in which AIF measurements are not possible. Improvements in the reproducibility of DCE-MRI model parameters (Ktrans, ve, and vp) were observed when this new, high-temporal-resolution population AIF was used, indicating the potential for increased sensitivity to therapy-induced change. Magn Reson Med, 2006. © 2006 Wiley-Liss, Inc.
-
https://www.rsna.org/uploadedFiles/RSNA/Content/Science_and_Education/QIBA/DCE-MRI_Quantification_Profile_v1%200-ReviewedDraft%208-8-12.pdf



